
The best time to see bladder cancer is before you can
even see it.
UroAmp MRD is a non-invasive genomic urine test that can reliably detect, monitor, and predict the risk of urothelial cancer or its recurrence well before signs and symptoms develop or become detectable by historical standards of care.
The test deeply interrogates 60 of the most high-impact urothelial cancer genes while broadly measuring changes across the whole genome. This in-depth approach has been validated to determine minimal residual disease (MRD) in patients with a history of bladder cancer following definitive treatment and/or before and after therapeutic intervention to monitor the efficacy of therapies in eliminating residual disease.
Each UroAmp MRD report provides a unique fingerprint of the patient’s cancer and cancer risk. These actionable insights enable unique data-driven decision-making that can help you better anticipate, monitor and personalize the care management for patients.
Unprecedented
Performance
Detects 92% of tumor mutations from urine DNA1
92%
OF TUMOR
MUTATIONS
Low-grade: Cytology 12.5% vs. UroAmp 67%
High-grade: Cytology 50% vs. UroAmp 100%2
SENSITIVITY
Identifies mutations for FDA-approved therapies erdafitinib and pembrolizumab
FGFR3
AND MSI
Unprecedented Performance
Can predict urothelial cancer months to years ahead of clinical diagnosis3
MONTHS TO YEARS
AHEAD
OF CLINICAL
DIAGNOSIS
![]()
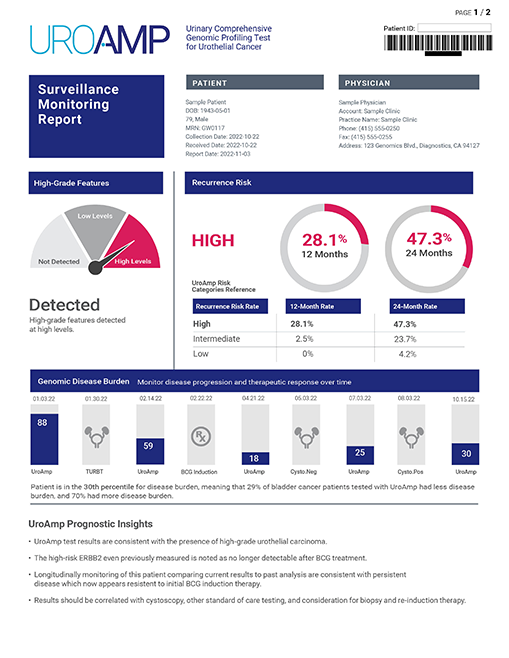
UroAmp MRD Report
This report provides a Positive or Negative output as well as a genomic disease burden (GDB) score and tumor grade prediction. The GDB is a measure of a patient’s disease burden compared to bladder cancer patients previously tested with UroAmp. UroAmp MRD can help monitor disease progression, recurrence, and response to therapeutic interventions.
Enhanced
Preservation Media
Urine is a hostile environment that can rapidly degrade and destroy DNA at room temperature. Unlike other labs, the UroAmp platform does not require labor-intensive storage and shipping requirements. Instead, we have developed a patented Enhanced Preservation Media (EPM) that stabilizes and preserves DNA in urine samples for up to 14 days, even at room temperature. Our EPM solution ensures minimal impact on clinic workflow and consistent, high-quality results are delivered for every patient.1
Contact us to learn more about how our solution can support your lab process.



